Ch 2.1 Reading Guide – Chemical context of life
OBJECTIVE: Understand that all organisms are made of matter, which is made of atoms.
- Which FOUR atoms make up 96% of all matter found in living organisms?
-
What is matter? (you may need to refer to your chemistry book or use internet)
-
What is the relationship between elements and compounds?
OBJECTIVE: Demonstrate how the structure of an atom determines its properties (mass, charge, ability to interact/bond with other atoms).
- The three main subatomic particles (parts of an atom) are:
- Which 2 of those three parts are in the atom’s nucleus?
- Describe the general structure of an atom and draw a sketch that shows the relative locations of each subatomic particle (label each part).
- Which subatomic particles have atomic mass of ~ 1 Da?
- Which subatomic particle has atomic mass of ~ 0 Da?
-
Using the periodic table, complete the table below and draw the structure of each atom with the appropriate number and location of protons, neutrons and electrons (assume each atom is neutral).
Carbon Oxygen Hydrogen Nitrogen Atomic num # Protons Atomic mass # Neutrons # Electrons - Draw each of the atoms in the table above here:
- Electrons are very small. How do you think you could determine how many there are in an atom?
- The atomic # always = # of
- How do you think the # neutrons could be determined?
-
This table contains different isotopes of carbon (12C & 13C) and an ion (13C-). Complete the table below:
12C 13C 13C- # Protons # Neutrons # Electrons OBJECTIVE Describe how valence electrons affect the ability of an atom to form bonds.
- What structural part of an atom are important in understanding how different elements behave?
- What are valence electrons?
- Look at the model of Neon.
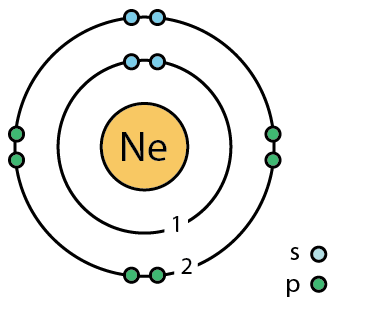
- How many electrons does the first electron shell hold?
- How many electrons does the second electron shell hold?
- What can you say about the reactivity of neon?
- How many valence electrons does nitrogen have?
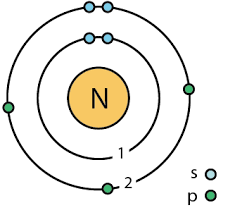
- How many valence electrons would make it stable?
OBJECTIVE: Understand how covalent bonding holds molecules together.
- What is a covalent bond?
- How does a covalent bond hold atoms together?
-
Draw 2 atoms of Hydrogen (which has 1 proton and 1 electron each). Show how they can bond together covalently. Draw any shared electrons in the space between the nuclei. Do the same for water. Use Fig 2.10 in your textbook if you need help.
OBJECTIVE: Understand how ionic bonds hold compounds together
- What is an ionic bond? How is it different from a covalent bond?
- If the two atoms aren’t sharing electrons in an ionic bond, what holds the two atoms together?
-
Draw a sodium atom and a chlorine atom side-by-side on your page. Then transfer one electron from the sodium atom to the chlorine. Which atom has more electrons than protons? Which has more protons than electrons? Indicate the charge on each.
OBJECTIVE: Define electronegativity of an atom and the role of electronegativity in bond formation.
- What is electronegativity?
- The following periodic table shows electronegativity. Mark an arrow on the side showing which way electronegativity increases as you go down the table (within a column (a group)). What physical attributes of the atom causes this trend?
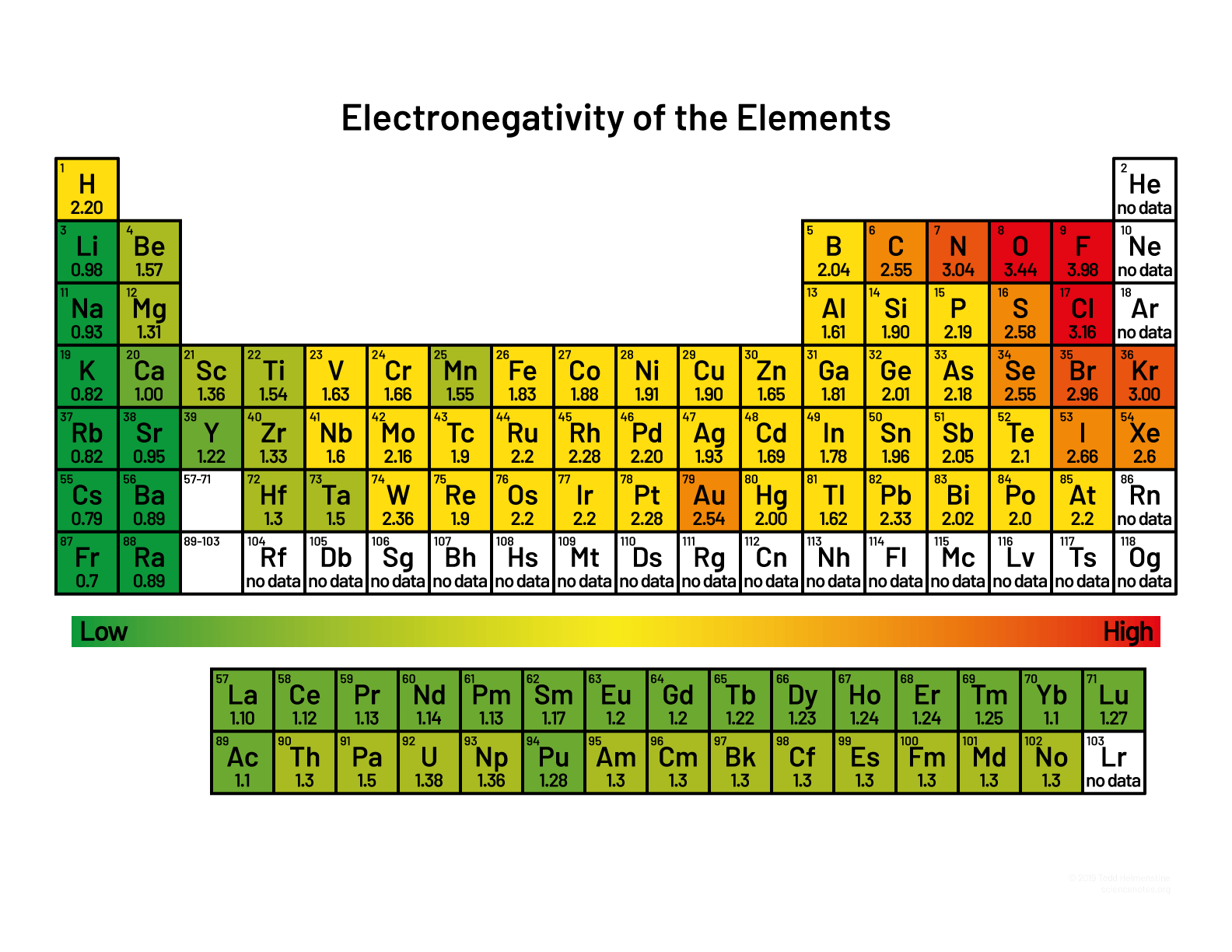
- Draw an arrow across the top of the periodic table above to indicate the direction in which electronegativity increases. What physical attributes of the atom cause this trend?
-
Which corner of the periodic table has the highest electronegativities?
OBJECTIVE: Understand the difference between polar and non-polar covalent bonds. Understand that hydrogen bonds result from polar covalent bonds
-
Draw a water molecule, showing all the valence electrons. Note that the 3-dimensional structure of water is non-linear.
OBJECTIVE: Understand that all molecules have a 3D shape that determines how molecules interact with other molecules.
- What determines a molecule’s shape?
- What is the 3-dimensional shape of methane that you drew above?
-
Why are the three atoms in water (H–O–H) NOT linear whereas the three atoms of CO2 (O=C=O) are arranged linearly. Draw all the valence electrons in your answer.
OBJECTIVE: Understand how carbon forms diverse covalent molecules
- Carbon has _______ valence electrons and will therefore form ______ covalent bonds.
- In a single covalent bond, carbon shares ______ of its electrons with another atom. In a double bond, it shares _______ of its electrons, and in a triple bond, it shares _______ of its electrons.
Do the following activities in or after class…
- Draw a carbon atom, showing ALL electrons (not just valence electrons).
- Draw hydrogen gas (H2) with all the electrons. Show how the covalent bonds form.
- Draw MgCl2, showing valence electrons. Show the transfer of electrons in the ionic bonds.
- Why does electronegativity increase from left to right across the periodic table?
- Why does electronegativity increase from the bottom to the top of the periodic table?
- Draw a water molecule with all valence electrons.
- Use the periodic table above to write down the electronegativity of each of the atoms in the water molecule.
- Which atom(s) is/are more electronegative? These atoms have extra attraction for the electrons. Mark this atom(s) with a (δ-) to indicate that the electrons are closer to this atom(s) and it/they are therefore carrying a partial negative charge.
- Which atom(s) are less electronegative? Mark them with a δ+ to indicate a partial positive charge.
- Now draw a second water molecule close to the one above. Use a dotted line to indicate a hydrogen bond between the partial positive charge on one molecule and the partial negative charge on the other. What forces draw the two molecules together?
- Draw a molecule of methane (CH4). The electronegativity of carbon and hydrogen are not identical, but similar. Why does methane not form hydrogen bonds with other methane molecules?
- Draw the structural formulas for C2H6, C2H4 and C2H2. Draw a bond as a single line representing a pair of electrons. Connect the Carbon atoms of C2H4 with 2 lines representing 2 pairs of electrons. Figure out the last one yourself. For each, count the number of valence electrons. Each bond contributes 1 valence electron to each atom being joined.
- Using hydrocarbons as an example explain what structural isomers are and draw two structural isomers of carbon.
- If time allows, use a modelling kit to assemble a model of methane. How is this shape different from what we draw on paper?
- Change your model so that there are 4 different atoms attached to the central carbon. Make a second molecule that is the mirror image. These are called enantiomers. Show that they cannot be superimposed and are therefore different, despite having the same atoms.
-
Look at figure 6.1 What feature makes saturated and unsaturated fats different?
OBJECTIVE: Recognize that chemical groups are responsible for most chemical and physical properties of organic molecules.
- You should be familiar with each of the functional groups listed in Table 2.3