Chapter 8 – Energy and Enzymes (8.1-8.2)
OBJECTIVE: Distinguish between different types of energy and describe how energy is transformed in chemical reactions.
- Distinguish between thermal and kinetic energy.
- How is chemical energy a form of potential energy?
- What is a chemical reaction?
- Explain the changes in kinetic and potential energy as a diver climbs a ladder and then dives.
- Use your own words to describe the second law of thermodynamics. Don’t use the word “entropy”.
-
Given your answer about the 2nd law of thermodynamics above, how can plants, for example, get away with decreasing entropy to assemble sugar molecules from Carbon dioxide?
OBJECTIVE: Understand how the change in Gibbs Free Energy indicates whether a reaction will be spontaneous.
- What is the definition of ‘enthalpy’ (H) (in about 4 words)?
- What is another word for Entropy (S)?
- What is Gibbs free energy (G)?
- Remember that Δ means “change”, and for now, ignore the T in the equation ΔG = ΔH - TΔS. Explain this using the simple definitions you wrote above.
- Spontaneous reactions have a (positive / negative) sign of ΔG and are called _________________
- Non-spontaneous reactions have ________________ sign of ΔG and are called ________________
- What is the sign on ΔS if entropy increases? _________________
-
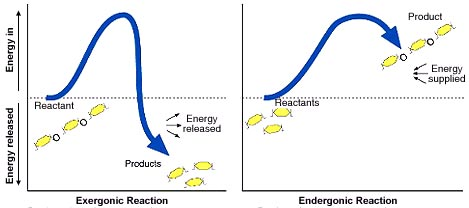
Explain the following graph: Describe whether the reactants or products have more energy. Do the reactions require energy to be supplied or do they produce energy? Which do you expect to be spontaneous? In which reaction is entropy increased? Decreased?
OBJECTIVE: Understand how temperature and concentration affect the rate of chemical reactions.
- Use the motion of molecules to explain how temperature affects chemical reactions.
-
Use the motion of molecules to explain how the concentration of reactants affects chemical reactions.
OBJECTIVE: Understand that redox reactions involve a transfer of electrons and that reduced compounds usually have high energy.
- A molecule that gains electrons has been ____________
- A molecule that loses electrons has been ____________
- Comparing FAD and FADH2, which is the reduced form?
- Look at the ‘gears’ in Fig 8.7. Which is the higher-energy compound, FAD or FADH2?
- Given your answer to the question above, is the conversion of FAD to FADH2 spontaneous or non-spontaneous?
- How is the reaction of FAD to FADH2 driven.
- Which of NAD+ and NADH is the reduced form?
-
Which of NAD+ and NADH has higher energy?
OBJECTIVE: Explain how nonspontaneous reactions may be driven using chemical energy (e.g. ATP) and energetic coupling (see Figure 8.10).
- Draw a cartoon of the structure of ATP. Indicate which part hydrolyzes to form ADP.
- What macromolecule from Chapter 5 does ATP look like?
- The figure below is from your textbook.
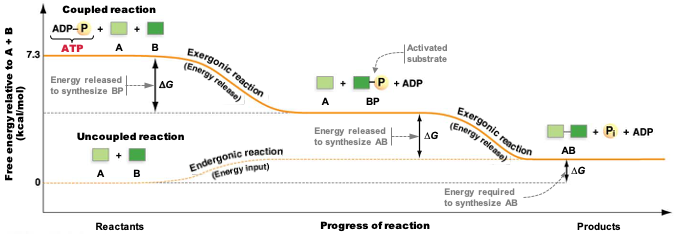
- What is the dependent variable in this figure?
- What is the reaction that this figure describes?
- Is the reaction endergonic or exergonic?
- Is the reaction spontaneous or non-spontaneous?
- What role does ATP play here?
- How does ATP help make this reaction exergonic?
- What organelle is primarily responsible for generating ATP?
- What is the relationship between ATP and ADP? Draw the cyclic reaction between the two. Which are endergonic and which are exergonic?