Chapter 8 – Energy and Enzymes (8.4-8.5)
OBJECTIVE: Describe how enzymes speed up metabolic reactions by lowering activation energy barriers.
- What type of molecule is an enzyme?
- What is the function of an enzyme?
- What is the activation energy?
- Why do reactants have to overcome the activation energy, even if the reaction is spontaneous?
- What types of bonds “hold” a substrate in the enzyme’s active site? DRAW a diagram that illustrates this concept.
- What is happening in the transition state?
-
Describe how an enzyme lowers the activation energy of a reaction (see p. 156)
OBJECTIVE: Describe the “Induced fit” model for enzyme activity and how it differs from the “Lock and Key” model.
- What is the name of the location on the enzyme that binds the substrate?
-
Compare and contrast the “induced fit” model with the “lock and key” model of enzyme activity. In your description, describe the rigidity of the enzyme and at what stage the enzyme binds most tightly to the substrate. Which is the more modern model?
OBJECTIVE: Understand what conditions can change the rate of a catalyzed reaction. OBJECTIVE: Connect the many factors that affect enzyme function to protein shape and the types of bonds involved.
- Most chemical reactions happen faster at higher temperatures. What does Fig 8.12 show that contradicts this for enzymatically catalyzed reactions? Why does this happen to enzymatically catalyzed reactions but not with non-catalyzed reactions? To answer this, think of what an enzyme is and what happens at high temperatures.
- Why does changing the pH of a reaction affect its speed?
- Why does the rate of a catalyzed reaction increase as the substrate concentration increases?
-
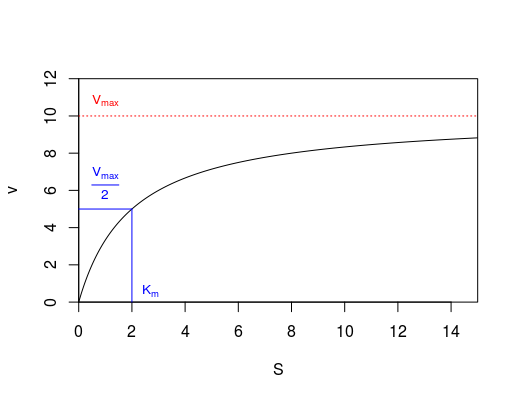
Look at the attached graph. Why does the rate of a catalyzed reaction plateau? (V is the reaction speed (“velocity”), S is the substrate concentration)
OBJECTIVE: Use diagrams to understand the difference between competitive and allosteric inhibition.
- Describe how competitive inhibition and allosteric inhibition work. Use a diagram to help.
Do the following in or after class…
- What happens to the rate of reaction if you increase the concentration of a competitive inhibitor? Why?
- What happens if you increase the substrate concentration in the presense of a competitive inhibitor?
- What happens to the rate of reaction if you increase the concentration of an allosteric inhibitor? Why?
- Look at Fig 8.19. What happens to the rate of production of the ‘product’ if there is a lot of ‘product’ present?
- Do you think Enzyme 1 would be active or non-active if enzyme 3 did not function properly? Why?
- Why do you think feedback inhibition is a good mechanism for controlling the rate at which a biological product (such as ‘product’ in Fig 8.19) is produced?